MASA. Since the orange menace ends his incoherent posts with "MAGA," apparently I should start them with a slogan (as per all posts in the Third Reich that started with two unmentionable words) and a good one is MASA - Make America Sane Again. And you can make TACOs with that! Also, in ongoing international news, remember that it is the duty of all nations to act to end a genocide and so far, only Yemen has answered the call.
Three parts to this issue – Economy and default, how elements form in stars, and a bit about possibilities of nuclear war.
The entire world financial system is being blown up by the orange menace and associated idiots, but perhaps BRICS+ will have another system up and running in time, before the total collapse of the Empire. The menace hopes to cut the Fed's interest rate to below 1%, which is actually a soft-default, bordering on hard default, since it severely diminishes the interest paid on USTs, the main national debt, which also takes down the value of most nation's currency reserves. Adam Posen (an important economist, BTW) was being interview on Paul Krugman's Substack and said this about the 3% rate cut:
"Three full percent is the kind of cut you do when it's 2008 and the world is coming to an end and you're trying to stabilize the world from coming apart. It basically requires not just a recession, but a belief of deep financial fragility in the system to justify that kind of change in monetary policy from anything that looks close to right."
The other soft-default action is the enactment of stablecoin, which forces ownership of USTs (which back stablecoin) into the hands of the general public (and banks), so that losses in USTs' value transfer onto the unsuspecting public. These (and disappearance of people) are banana-republic measures thus it won't be long before the entire country falls into social chaos, since even in "good times" it exists close to the edge of that abyss.
Back to nuclear physics for a few minutes! Hang in there and keep reading, guaranteed you will learn at least two things. I've been reading an article in Quanta Magazine, "Physicists Start To Pin Down How Stars Forge Heavy Atoms," by Jenna Ahart, July 2, 2025. Some of this article derives from that source, other parts from elsewhere.
First a little backgrounder on fission vs fusion. Fission is splitting of atoms, heavy fissile atoms, meaning atoms which can undergo fission after absorbing a slow or thermal neutron. The bomb dropped on Hiroshima was a uranium bomb made from enriched uranium, to contain a high concentration of 235U. The next bomb dropped on Nagasaki was a 239Pu plutonium bomb. There was a list of six possible cities for that second bomb. One of them was Kyoto, but one of the generals had a honeymoon there a number of years earlier, so the choice dropped to the last one on the list of populations to be incinerated to demonstrate the Bully's new toy: Nagasaki.
Fusion occurs when two lighter nuclei fuse together to form a heavier element's nucleus, but the resulting mass is slightly less since some of the mass was converted to energy. This is the energy source that keeps the Sun burning, and atoms of various elements can keep fusing together and giving off this energy, advancing along the increasing atomic weights of elements on the periodic table, until you get to iron (Fe). Beyond iron, fusion can continue, but there is no energy surplus; energy must be added to promote the reaction. So, you add energy to make the heavier elements, and they can give this energy back when breaking up, in fission reactions.
What is going on in the Sun? Every second, the core of the Sun fuses 620 million tonnes of hydrogen nuclei into 616 million tonnes on helium. The apparent mass loss is the amount converted to the energy that powers the Sun, 0.645% of the mass of the hydrogen nuclei converts to kinetic energy on the release of an alpha particle and other radiative energy such as electromagnetic radiation. Thus helium is the first step toward heavier elements. The most common isotope of helium is Helium-4, which should be written (superscript 4)(subscript 2)He, which I could do on a typesetter, but not in MS Word, so I will write it 4,2He, meaning 4 neutrons, 2 protons. A 3,1H (called tritium, a heavy isotope of hydrogen with 2 neutrons) and a 4,2He could fuse to make a 7,3LI (lithium, the lightest metal). Three 4,2He could fuse to make 12,6C, or 12C, meaning Carbon-12 (carbon always has 6 protons). The process continues, shown in this graphic:
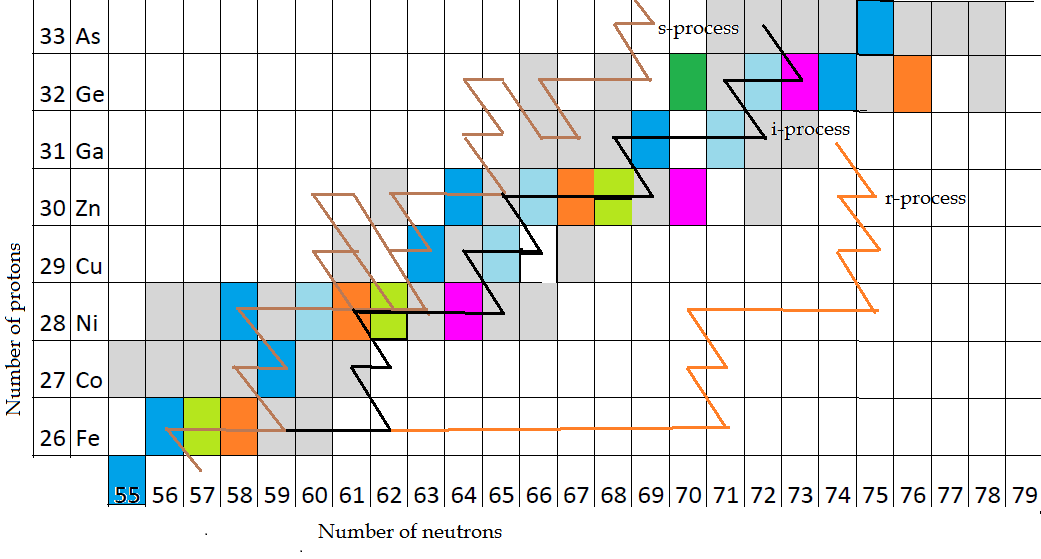
Explanation of this graph – The most common isotope of each element in terrestrial conditions is highlighted with the block filled with blue. Other terrestrial isotopes in smaller percentages are shown as other colours; all blocks without a colour, including grey, are radioactive. Grey blocks have short half-lives. Remember, these are terrestrial conditions, what goes on inside stars can be very different. This data from the IUPAC Periodic Table of Elements ©Sara Glidewell. The brown zig-zag lines represent the s-process of creating heavier elements, black represents the i-process and orange, the r-process. The brown line starts at the bottom, actually in the row below iron (Fe=iron) which is manganese, 25 protons, but it has gained two neutrons from fusion reactions in a star to become 57Mn (atomic mass of 57). Then you see the line rising up at an angle to the 56Fe blue block. That diagonal line represents a beta-decay – a neutron expels an electron and an antineutrino to become a proton – in that transition 57Mn became 56Fe. That stage represents the last exothermic fusion reaction in a star (contributing heat to the star). For the star to generate heavier elements than Fe means inputting more energy into the reaction than is gained, so it drains energy from the star. To carry on creating heavier elements, you now follow along travelling right from Fe along one of the s, i, or r processes. Moving along the line to the right, you are adding neutrons to the element, so the next step is add 3 neutrons to get 59Fe (s-process), 6 neutrons to get 62Fe (i-process) or 15 neutrons to get 71Fe (r-process). Then for each colour of line, you do a beta-decay to jump from 59Fe to 58Co (cobalt) in s-process; from 62Fe to 61Co (i-process); or 71Fe to 70Co (r-process). Then, moving up the chart, it all repeats, adding a neutron and doing beta-decays, to change 59Co to 58Ni, 62Co to 61Ni and 71Co to 70Ni. This process continues on above this chart, it shows just this portion as an example.
The s-process (slow process) takes thousands of years, primarily in asymtotic giant branch stars, with free neutron densities between 105 and 1011 neutrons per cc; i-process takes days to years in a setting still uncertain, with neutron densities ±1015 neutrons per cc; and r-process which takes seconds, possibly during neutron-star mergers, with extreme neutron densities ±1025 neutrons per cc.
A bit more on fusion - The fusion in a thermonuclear (hydrogen) bomb (an evil in this world) is an atom of 2,1H fusing with 3,1H (2,1H is also called deuterium and 3,1H is also called tritium) to yield 4,2He (helium) plus 1,0n (a free neutron) carrying 14 MeV (mega-electron volts) of energy or 1400 TJ/kg (terajoules per kg) or a speed of 52,000 km/s, 17.3% of speed of light. This reaction was first observed in 1938 by Arthur Ruhlig at U. of Michigan and is called "the most favorable reaction" (highest energy output).
But saying that they fused deuterium with tritium is typical tripe that they feed you. It was done another way, and this was a military secret for a long time. The Manhattan Project was a vast undertaking, and its cost still lingers unpaid in the national debt. What they actually used was a special metal hydride, made with 6Li (Lithium-6, the least-abundant natural isotope of Lithium) combined with deuterium, making 6Li2H. That was a lot of processing, to collect enough Lithium to extract a quantity of Lithium-6, when that is less than 8% of normal abundance. Same problem for deuterium (2H), very tiny amounts occur in nature. So they take two difficult-to-source elements and combine to form a lithium hydride 6Li2H (6Lithium deuteride) using a light lithium and a heavy hydrogen. This trick gets the 2H (deuterium) into the weapon in solid rather than gas form. This lithium deuteride is bombarded with neutrons converting the lithium to 4He (helium) plus a 3H (tritium). The tritium fuses with the deuterium that was attached to the lithium, providing "that most favorable reaction" creating 4He (Helium) and the huge release of energy. The outer shell of the bomb is lined with 238U, the most common form of uranium which is the leftover part from enrichment which values the smaller 235U fraction. 235U is fissile (easily undergoing a chain reaction) but 238U is fissionable, meaning it requires higher-energy neutrons than its fission produces. That is what the "most favorable reaction" is for! The high-energy neutrons arising from the deuterium-tritium fusion ignite the 238U resulting in the greatest part of the bomb's energy yield and most of its radioactive debris. The cheapness of both the lithium deuteride and 238U have allowed for very large nuclear arsenals. You can read more of this story on Wikipedia, you will see a lot more military secrets there, and have a more complete look into the depravity of humanity.
The sideshow here is that reagents containing the remaining depleted lithium (now having a higher concentration of 7Li than is found in nature) were sold to chemical manufacturers and laboratory chemists for their normal uses. This trick allowed monitoring of factory and lab waste pollution tracking in waterways, to determine if proper disposal methods were used, since if the lithium detected in waterways was shown to be depleted in 6Li compared to natural levels, the pollution source could be confirmed.
Rather than mainly the Bulletin of Atomic Scientists bringing forward articles about possibilities of nuclear war, Nature Science has a new article, "How to avoid nuclear war in an era of AI and misinformation, nuclear deterrence is no longer a two-player game, and emerging technologies further threaten the status quo. The result is a risky new nuclear age." by Alexandra Witze. An interesting part:
"David Gross, a physicist at the University of California, Santa Barbara, was inspired to help arrange the Chicago conference after nuclear weapons were barely discussed in many consequential elections in 2024. 'People are not really aware of the dangers' of nuclear war, Gross says. 'It is a very precarious situation.'"
The above link to The Bulletin is for an article from July 15th: "Introduction: Possible flashpoints for the next major conflict" by Dan Drollette Jr. It has several links to other articles on this topic.
References:
https://www.quantamagazine.org/physicists-start-to-pin-down-how-stars-forge-heavy-atoms-20250702/ Physicists Start To Pin Down How Stars Forge Heavy Atoms, by Jenna Ahart, July 2, 2025, Quanta Magazine.
https://iupac.org/iptei/
How to avoid nuclear war in an era of AI and misinformation, Nuclear deterrence is no longer a two-player game, and emerging technologies further threaten the status quo. The result is a risky new nuclear age. By Alexandra Witze, July 17, 2025, Nature 643, 898-900 (2025) https://doi.org/10.1038/d41586-025-02260-z
Leave a comment! This is a re-direct to my Substack page.
Return to Limits to Progress Main Page
If you would like to send a donation, please send an Interac eTransfer to email address below. Thank you!
©2025 Kathleen McCroskey
Send your e-mail comments and questions to: