Physics review: 3NF and the shape of 208Pb
Lead
Sometimes I want to write about something that interests me,
rather than keep harping on existential threats to this failing
species, even though this site is probably more effective in
identifying those threats than the Cambridge Centre for the Study
of Existential Risks, CESR.
So today's topic is sub-atomic particles. I've been working on
this article since January 31, but every few days new astonishing
research reports come in rewriting major theory on atomic
structure. So, after people pick their jaws back up off the
floor, back to rethinking almost everything. Then a few days, and
it happens again. First was a discovery of a third binding force
in the nucleus of atoms. Then a bit later, why a 208Pb (lead)
atom is not round as previously thought.
Wait, don't leave yet!
This isn't too difficult, this is just about the occupants of the
nucleus, protons and neutrons, collectively known as
"nucleons." But yes, this is about stretching your brain a
bit, so no pain, no gain; lean in there and read! Learning some
new things makes some new neural connections in your brain which
might be useful later on. Keep reading! I know you can
read more of this than Trump can, as he leads us into the new
Dark Ages devoid of science.
Some definitions:
What's the difference between an atom and an element? An
atom is the smallest unit of an element and cannot be
further broken down chemically. An element is an atom with
a particular number of protons – no two elements have the
same number of protons. Each atom has an equal number of protons
(positively charged) and electrons (negatively charged) unless it
is in an ionized state. An atom of an element can have differing
numbers of neutrons (neutral electrical charge), each of
which represents a different isotopic nuclide. A
nuclide is an atom with a specific number of protons (its
atomic number) and a specific number of neutrons (its mass
number). The terms Nickel-64 and 64Ni both refer to a
nuclide of the element nickel with a mass number of 64. Nickel
has 28 protons and 36 neutrons, for a total atomic mass of 64.
For an example of an element with different number of neutrons,
you can have, for instance, Carbon-12, Carbon-13, or Carbon-14,
better written as 12C, 13C and
14C. Each of these is an isotopic nuclide, or
isotope. But every form of carbon has just six protons. If
you could add another proton, to make seven protons, it is no
longer carbon, it becomes Nitrogen! It changes from a dark
solid (or diamonds) to a gas!
Each element is an amazing thing, each unique in its
properties. Alchemists- here's your opportunity. You want to
change mercury into gold? Just take out one proton, now it
changes from a heavy metallic liquid into an expensive metal.
Between that last paragraph and the next, I've been off
about a week with a no-fever, no-oxygen covid-type illness, so
stop licking your screen and wash after reading. Or burn after
reading.
Look around at everything around you – it is all made of
these tiny bundles of protons and neutrons (nucleons) with
electrons swirling around them. Is a hydrogen proton the same as
an iron proton? Apparently. And amazing forces hold them
together, so that iron can be strong and hard. Imagine if you
stepped on some carbon, such as graphite, and the atoms broke
apart because the forces were weak. Two of the 6 protons could
fly off free, along with an electron, and float away as hydrogen.
The four remaining could go off as two pairs of two protons, and
float away as helium. That would be a dangerous world, with
nothing holding together in the way you are used to.
In this example of broken carbon, I didn't mention the
neutrons.
Why does carbon have 12, 13 or 14 neutrons, and not 2 or 24? It
is possible that it could, but usually we're talking about
terrestrial conditions (on Earth), not about the weird things
that go on in creation of elements in stars. On Earth, other
nuclides of carbon are unstable, they decay, fall apart.
In the IUPAC Periodic Table of
Elements and Isotopes, Carbon is shown with 15 isotopes - all
of course with 6 protons, but neutrons varying from 8 to 22.
Number 8, 9, 10, 11, 15, 16, 17, 18, 19, 20, 21 and 22 have
half-lifes of less than an hour. Two stable isotopes are
12C (about 99% of carbon) and 13C (about
1%). 14C has a very long half-life and is used in
carbon dating of fossils.
But why any neutrons at all? The Coulomb force between protons
(their positive charge) would push them apart, while adding
neutrons helps cement them together in the nucleus. There is, for
each element, certain values in the collection of protons and
neutrons that result in varying degrees of stability of that
isotopic nuclide.
So what holds atoms together?
In the old books, the four fundamental forces of Nature were
considered to be gravity, the weak force, electromagnetism and
the strong force. But we need to move beyond that.
The Strong Force binds quarks together to form the protons
and neutrons, while the Weak Force involves certain forms
of nuclear decay, involving W and Z bosons. The Two–Nucleon
Force is an attraction between two nucleons (a proton and a
neutron). It attracts them at long range and repels them at short
range, keeping a certain distance between them. These
interactions keep the nucleus stable and in a low energy state.
It allows for the nucleons to be arranged in "shells," like one
hollow ball inside another. Full shells occur at 2, 8, 20, 28,
50, 82 and 126 protons or neutrons, so actual shell nucleon count
is 2, 6, 12, 8, 22, 32 and 44 (subtracting an above number from
the previous one), so if you add together this last series, it
comes to 126. The number of protons or neutrons in these amounts
is called a "magic number." Therefore, tin (Sn) at 50
protons has a "magic number" of protons. And lead (Pb), which we
will get to in more detail below, is "doubly magic"
because it has a magic number of protons (82) and a magic number
of neutrons (126).
In the two-nucleon force, the two nucleons interact by tossing a
meson between them, with the lightest meson being a
pion, responsible for the long-range attraction between
nucleons.
But new research (published August 2024), has brought another
factor into view, the Three-Nucleon Force (3NF), in work
published by lead author Tokuro Fukui at Kyushu University,
Japan.
In the review from phys.org:
"Researchers from Kyushu University, Japan have
revealed how a special type of force within an atom's nucleus,
known as the three-nucleon force, impacts nuclear
stability.
Understanding how these nucleons interact to keep the nucleus
stable and in a low energy state has been a central question in
nuclear physics for over a century. Now, this new study clarifies
the mechanism of how the three-nucleon force (3NF) enhances
nuclear stability, and demonstrates that as the nucleus grows,
the force gains in strength."
As the 3NF grows in strength in heavier nuclei, it increases
nucleus stability by increasing the energy gap between the shells
of nucleons. This is achieved by spin-orbit splitting (oops,
did I just lose some readers? Keep reading!). These
little nucleons (protons and neutrons) have intrinsic spin, they
are always spinning! If they spin and orbit in the same direction
in the nucleus, the result is a lower energy level. A lower
energy level in a nucleus means being in a lower (energy-level)
shell. When they spin and orbit in opposing directions, they are
in a higher energy state, thus "split" into a higher energy
shell, providing the nucleus with a more stable structure. Here's
an image of nuclear shells. 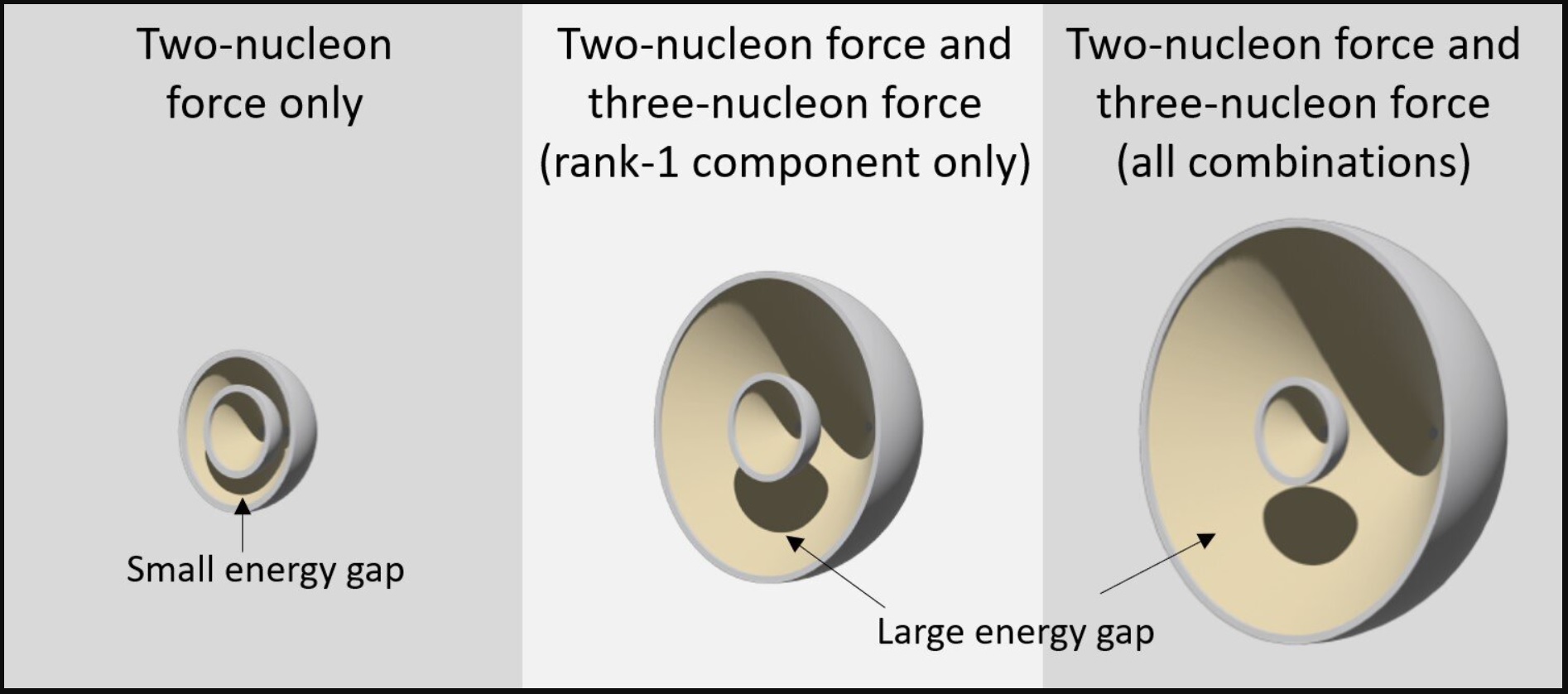
(Image credit: Prof. Tokuro Fukui, Kyushu
University)
In the 3NF, three
nucleons are tossing two mesons around between them. In this
exchange of two mesons between three nucleons, the nucleons are
constrained in how they move and spin, with only four possible
combinations, one of which is called the "rank-1 component" which
plays a critical role in nuclear stability. And in another
discovery, they found that while in the two-nucleon force, you
can measure the spin-states of the two nucleons individually, in
the 3NF, quantum entanglement occurs, in which two of the three
nucleons can have spins in both sates at once, until you try to
measure them. (The cat is both alive and dead)
When you look through a pin-hole in science into this world of
particle physics, you see this remarkable level of human
intelligence at work, in a depth and complexity almost
unimaginable. See an example of one of the mathematical formulas
from Dr. Fukui's paper here: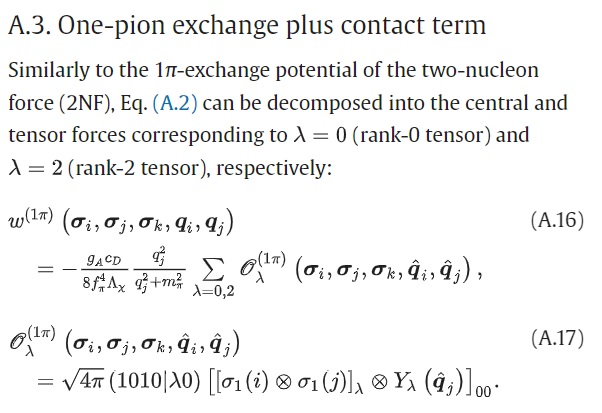
Or, open on the
original paper, find in the left side-bar "Appendix A" and
click on that.
See, I'm trying to greatly simplify this material so that you
might be able to understand it, so please keep reading!
But, shockingly, when you pull your head back out of the science
world of nuclear particles, and look around, you see the massive
chaos created by ignorance and stupidity in this failing world
order.
Then the next report that changes everything - the Pb (lead)
nucleus is not round, as expected, it is more like a rugby ball.
You can d/l the PDF of the original paper
here.
Some quotes from the
phys.org report:
"Dr. Jack Henderson, principal investigator of the
study from the University of Surrey's School of Mathematics and
Physics, said, 'We were able to combine four separate
measurements using the world's most sensitive experimental
equipment for this type of study, which is what allowed us to
make this challenging observation. What we saw surprised us,
demonstrating conclusively that lead-208 is not spherical, as one
might naively assume. The findings directly challenge results
from our colleagues in nuclear theory, presenting an exciting
avenue for future research.'
Theoretical physicists, including those at the Surrey Nuclear
Theory Group, are now re-examining the models used to describe
atomic nuclei, as the experiments suggest that nuclear structure
is far more complex than previously thought.
An international research collaboration led by the University of
Surrey's Nuclear Physics Group has overturned the long-standing
belief that the atomic nucleus of lead-208 (208Pb) is
perfectly spherical. The discovery challenges fundamental
assumptions about nuclear structure and has far-reaching
implications for our understanding of how the heaviest elements
are formed in the universe."
Here is a link to a
one-page 1965 letter to the journal Nature telling
about Magic Numbers, you can click the link to the PDF and print
it out to hang on your wall, to have something written by Linus
Pauling which isn't about Vitamin C.
References:
• PAULING, L. Structural Basis of Neutron and Proton
Magic Numbers in Atomic Nuclei. Nature 208, 174 (1965).
https://doi.org/10.1038/208174a0
• Tokuro Fukui, Giovanni De Gregorio, Angela Gargano,
"Uncovering the mechanism of chiral three-nucleon force in
driving spin-orbit splitting" Physics Letters B Vol. 855 (2024)
138839
https://www.sciencedirect.com/science/article/pii/S0370269324003976?via%3Dihub
https://dx.doi.org/10.1016/j.physletb.2024.138839
• Henderson et al. "Deformation and Collectivity in
Doubly Magic 208Pb" Physical Review Letters, 134, 062502 (2025)
https://doi.org/10.1103/PhysRevLett.134.062502 (courtesy of
Creative
Commons Attribution License 4.0)
• Zsolt Sóti, Joseph Magill, Raymond Dreher,
"Karlsruhe Nuclide Chart – New 10th edition 2018," EPJ
Nuclear Sci. Technol. 5, 6 (2019)
https://doi.org/10.1051/epjn/2019004
https://nucleonica.com https://www.epj-n.org
• IUPAC Periodic Table of the Elements and Isotopes
(IPTEI) for the Education Community – Update 2019 (IUPAC
Technical Report) by Norman E. Holden, Tyler B. Coplen, John K.
Böhlke, Lauren V. Tarbox, Jacqueline Benefield, John R. de
Laeter, Peter G. Mahaffy, Glenda O'Connor, Etienne Roth, Dorothy
H. Tepper, Thomas Walczyk, Michael E. Wieser, and Shigekazu
Yoneda https://iupac.org/iptei
Leave a comment! This is a re-direct to my Substack page.
Return to Limits to Progress
Main Page
If you would like to send a donation, please send an Interac
eTransfer to email address below. Thank you!
©2025 Kathleen McCroskey
Send your e-mail comments and questions to:
Mail page

